Ingredients for Society
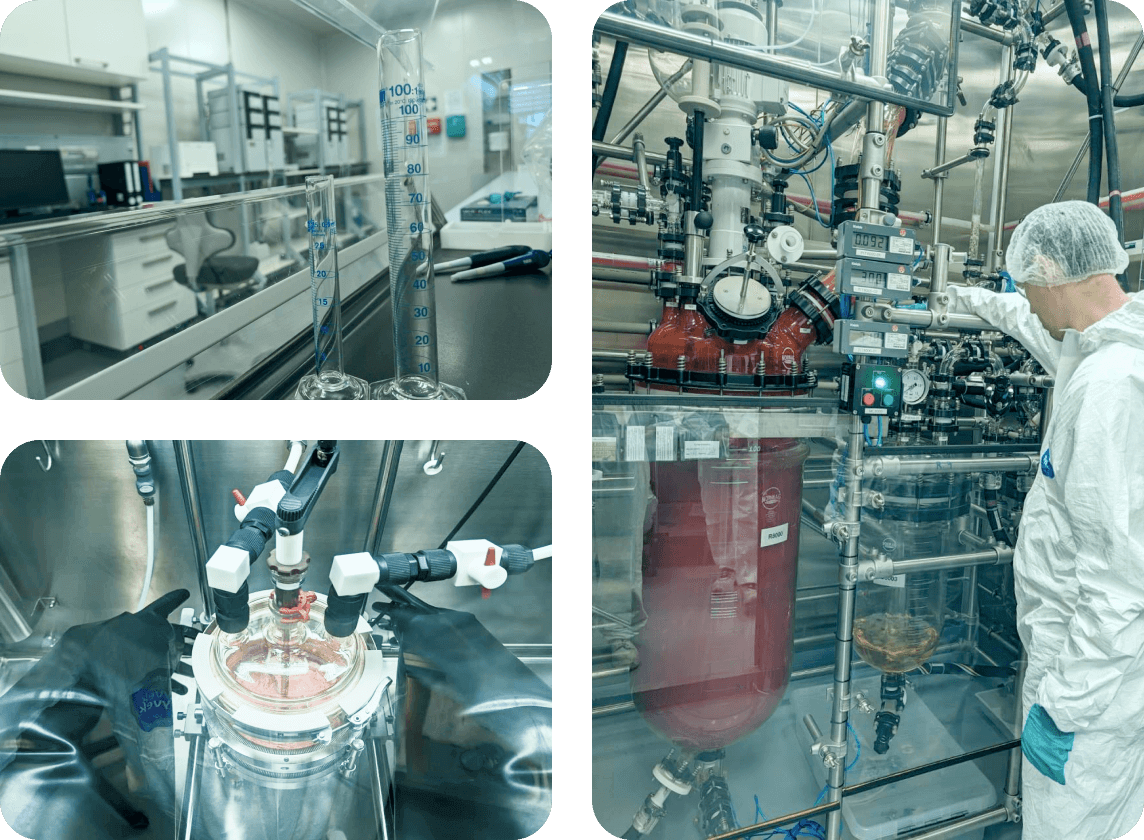
Our primary specialization lies in developing and manufacturing highly potent
Active Pharmaceutical Ingredients (APIs), primarily used in cancer therapies.
Company
Our mission is to advance global health
The Synbias-Gemini Group operates under the umbrella of Synbias Pharma AG, headquartered in Schaffhausen, Switzerland, which 100% shareholder of Gemini PharmChem Mannheim GmbH, our production facilities, a highly esteemed manufacturing and R&D site located in Mannheim, Germany.
Read moreof the world market of particular API 21 countries
Our APIs are shipped to customers in 21 countries
Successfully passed through FDA, Anvisa, cGMP and others
Inspections
Compliance You Can Trust
At Synbias-Gemini Group, we have a distinguished track record with global regulatory agencies, demonstrating our unwavering commitment to compliance and quality. Our collaborative approach ensures robust support to our customers through all phases of regulatory submission, ensuring that every product we manufacture meets the highest standards.
Inspection History (2012-2024)
- Germany Regierungspräsidium Tübingen (German authority): 8 inspections
- US FDA: 1 inspection



Products
Our technology allows us to be competitive
At Synbias-Gemini Group, our research and development pipeline is dedicated to advancing cancer treatments through the development of innovative and highly effective active pharmaceutical ingredients (APIs). These efforts are backed by our state-of-the-art facilities and a team of highly skilled scientists committed to excellence.
Read More